Which of the Following Atoms Has the Greatest Metallic Character
Bismuth has the lowest rate of electronegativity and ionization energy thus it is more likely to lose an e- than the rest of the elements of the said group which is why it is. The element in Period 3 that has the greatest metallic character is magnesium.
Metallic Character The Periodic Table Of Elements
In periods the metallic character decreases when atomic number increases.
. Ultraviolet light 150nm Choose the element with the larger atoms from each of the following pairs. Which compound listed below will dissolve in. The metallic character of an element decreases as you move from left to right or bottom to top of the periodic table.
The comparison of the number of atoms in a copper coin the size of a penny with the number of people on earth is made to illustrate which of the following. Franium element with highest metallic character caesium next highest level of metallic character sodium. 3Li Z Li 3 Z.
Which of the following atoms has the greatest metallic character. Br Se Ni As Si. Up to 24 cash back Metallic character is a name given to elements that are metals and their chemical properties.
Which of the following atoms has the greatest metallic character. According to the periodic table metallic character increases down the group and decreases across the period. Chemistry questions and answers.
Hence option a is correct. So Cs has greatest metallic character. Which molecule listed below has a nonpolar covalent bond.
That atoms are very small c. Group 15 of the periodic table contains five elements beginning with. C X has greater metallic character than Y does.
A Al or In. YOU MIGHT ALSO LIKE. The element in Period 3 that has the greatest metallic character is magnesium.
Which of the following atoms has the greatest metallic character. N Nitrogen P Phosphorus As Arsenic Sb Antimony and Bi Bismuth. These two atoms have two valence electrons thus they have similar chemical properties.
Au QUESTION 10 Which of the following atoms is the smallest. Therefore Ba has the greatest metallic character. Determine which of the following kinds of light contain enough energy to break chemical bonds in biological molecules by calculating the total energy in 1mol of photons for light of each wavelength.
Francium is extremely rare and is radioactive with the longest half-life at 22 min so there is no empirical. The alkali metals in group 1 are the most active metals and cesium is the last element in the group for which we have experimental data. In periods the metallic character decreases when atomic number increases.
D X has a larger first ionization energy than Y does. Increasing order of metallic character is nickel Arsenicseleniumbrominesilicon. Bismuth is the element in group 15 that has the strongest metallic character.
Chem test 2 sem 2. What is the formula for the compound if we substitute sodium for lithium. Which of the following atoms has the least metallic character.
That atoms are very large d. See answer 1 Best Answer. Cs Be Cu Ti Au.
MCAT Mometrix Comprehensive Guide. All of the compounds none of the compounds e. This is because atoms have a better ability to gain electrons to fill a valence shell than lose them to and an unfilled shell.
This occurs as atoms more readily accept electrons to fill a valence shell than lose them to remove the unfilled shell. N O DCI OUESTION 11 QUESTION 11 Which of the following elements has the highest first ionization energy. That atoms are indivisible b.
C P or Pb. Some elements which shows metallic character are listed below. E X is a poorer conductor of electricity than Y when in the solid state.
Metallic character increases as you move down an element group in the periodic table. The arrangement of the group 15 elements in ascending rate of metallic character is. Therefore the correct option is d.
Consider the following reaction. B X has a larger effective nuclear charge than Y does. Which of the following atoms has the lowest ionization energy.
Which of the following elements has the greatest metallic character. The metallic character increases from right to left along a period and top to bottom in a group. A X has a larger electron affinity than Y does.
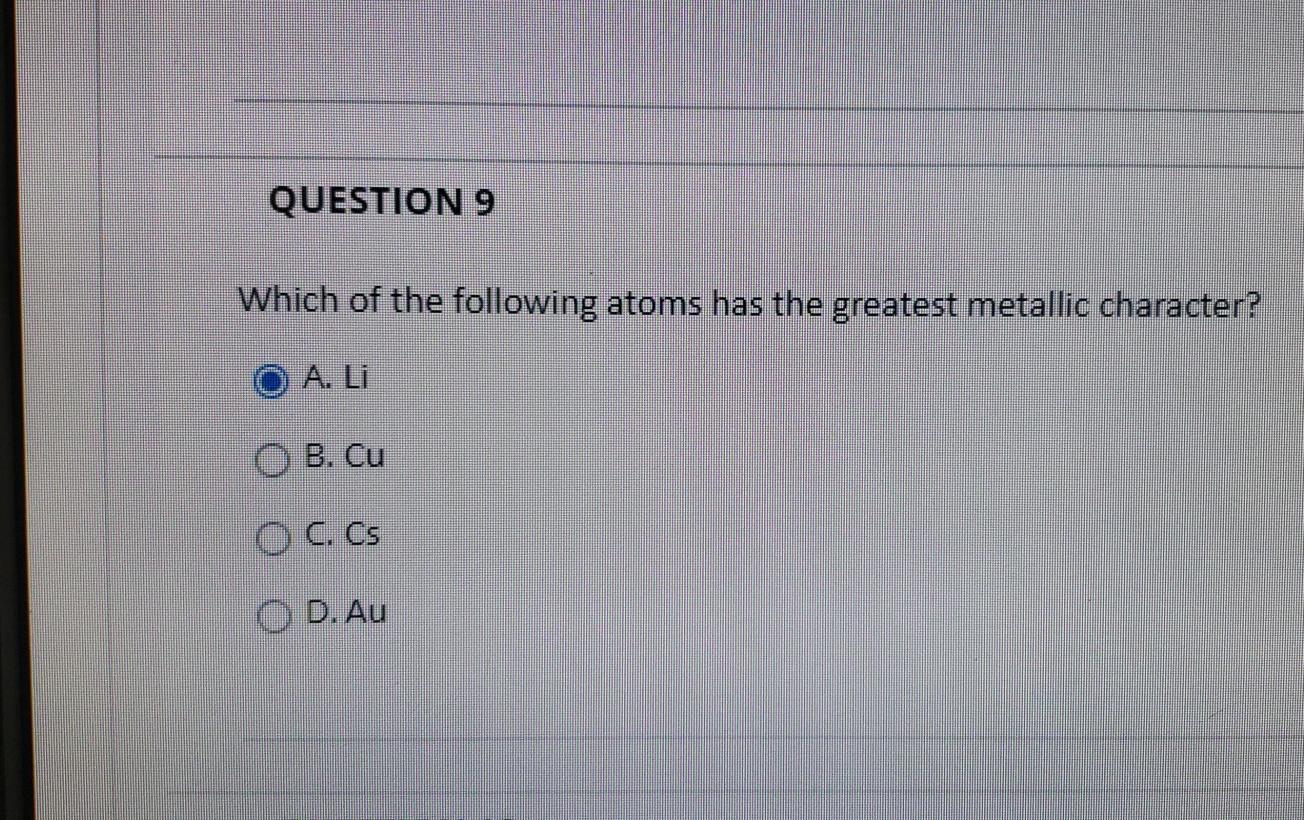
QUESTION 9 Which of the following atoms has the greatest metallic character. B Si or N. As Calcium and magnesium both belongs to the same group of the periodic table that is group 2.
Metallic character increases form right to left across a period on the periodic table and from top to bottom down a group. D C or F. That in a copper penny there is one atom for every person on earth.

Solved Which Of The Following Atoms Has The Greatest Chegg Com

Solved Question 9 Which Of The Following Atoms Has The Chegg Com

No comments for "Which of the Following Atoms Has the Greatest Metallic Character"
Post a Comment